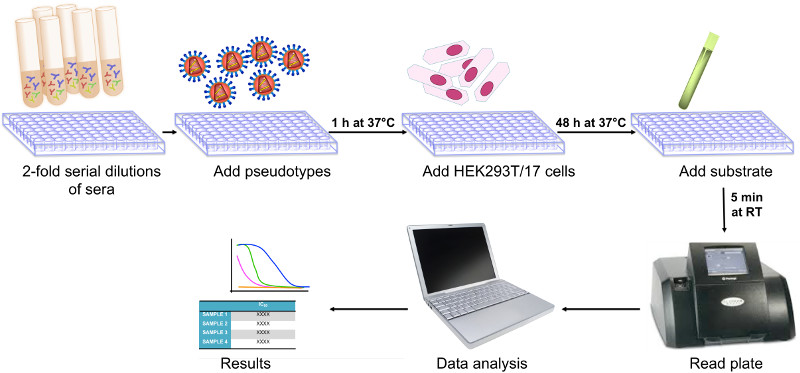
Influenza pseudotypes have been employed extensively by us to undertake serological assays for the vaccine industry, and could potentially contribute towards the licensure of influenza vaccines, at least as an adjunct assay. In a joint study with Novartis Vaccines, we showed that pseudotypes could be used to sensitively quantify neutralising antibody responses elicited by a pre-pandemic H5N1 vaccine and that these correlated significantly with those measured by haemagglutination inhibition (HI), single radial haemolysis (SRH) and microneutralisation (MN). We have made an optimised protocol available for undertaking such pseudotype neutralisation assays. The cartoon illustration below is taken from this protocol.
Pseudotype neutralisation assays for influenza have been shown to be exquisitely sensitive for the measurement of responses to the HA stalk, which is one of the primary targets of many ‘universal vaccine’ approaches. The responses directed against the stalk can be separated from those against the head by making use of a hybrid HA pseudotype assay. One such hybrid pseudotype virus that we have created has an HA stalk derived from the 2009 pandemic H1N1 strain, and an HA globular head derived from an H11 strain which is found in wild birds. Humans have negligible serological reactivity to H11, so if a neutralisation assay is undertaken with this hybrid HA pseudotype, one can readily measure the response directed against the HA stalk only.
The traditional HI assay (for which a correlate of immunity exists) used by the regulators only measures responses against the globular HA head and thus is not fit for purpose for the licensing of many new ‘universal vaccines’. HA stalk serological assays, such as those based on pseudotypes or ELISA, will no doubt gain prominence as many of these new vaccines move down the clinical pipeline.
For neuraminidase (NA), a pseudotype-based enzyme-linked lectin assay (PV- ELLA) has been developed, which allows the quantification of antibody responses against NA. This assay innovatively uses NA-only pseudotypes as a source of NA. A fully detailed PV-ELLA protocol is available here. The availability of this PV-ELLA to the R&D community is instrumental as it means that ELLA assays can be performed without requiring access to reverse genetic (RG) viruses, which often have IP attached. Also, there are moves towards standardising the amount of NA in recombinant vaccines, making PV-ELLA assays increasingly important.
Note: We previously also co-developed and tested a cell-based PV release assay for NA inhibitors which is published here.