Work from the School of Biosciences has been highlighted by the Journal of Biological Chemistry in a special virtual issue.
This collection of articles explores the polymers and motor proteins that determine the function of the eukaryotic cytoskeleton in cell migration, cardiac health and neurodegeneration. The editors looked through a large number of papers to come up with what they felt best represented the exciting advances in this field since 2018. The collection includes two papers from the Geeves laboratory.
The first, a joint Kent – USA collaboration supported by the British Heart Foundation and the US National Institutes of Health tries to understand the causes of inherited heart disease in order for treatment to match the cause of the disease and not the symptoms. Five different mutations in the human heart protein myosin were studied; each altered the protein in a different way but each resulted in a similar defect in the heart. Each of these mutations therefore may require a different treatment. (Dilated cardiomyopathy myosin mutants have reduced force-generating capacity. First published in April 2018).
The second, a collaboration between the universities of Kent, Illinois and Colorado, funded by the US National Institutes of Health and the EU, was the PhD work of a Kent student Chloe Johnson. This measured the differences between seven different forms of the protein myosin that define the contraction of seven different types of human muscle including atrial and ventricle heart muscle, and the fast and slow skeletal muscles. Each myosin is slightly different and matched to its role in the body. (The ATPase cycle of human muscle myosin II isoforms: Adaptation of a single mechanochemical cycle for different physiological roles. First Published August 2019).
Congratulations to Prof Geeves and all those who contributed to this outstanding work.
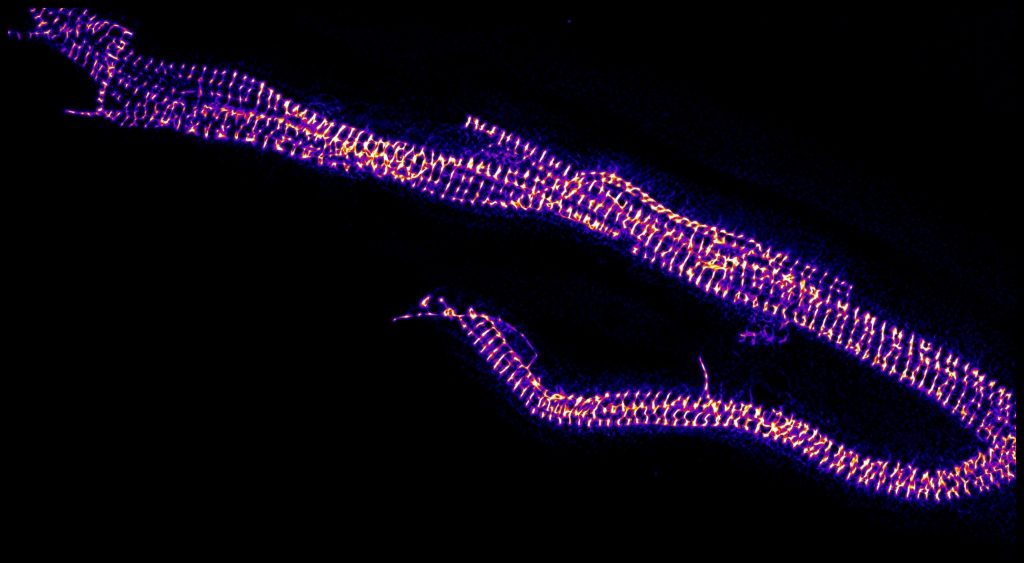
Image: muscle myofibrils stained with a fluorescent dye to highlight the repeating banded pattern of the fundamental structural unit of muscle, the 2.2 micrometer long sarcomere.