Protein-based drugs, such as antibodies, are extremely effective for the treatment of a number of diseases including diabetes and cancer.
These complex molecules are usually produced in mammalian cells grown in culture, as only these cells have all the cellular machinery required to produce proteins with essential human-like attributes. A new research paper from the Smales Lab in collaboration with Lonza Biologics shows how genetic engineering can be used to modify the lipid content of the cell “factories” to improve production yields. Lipid membranes are crucial in many cellular processes including growth, proliferation, and protein synthesis.
Dr James Budge (a lead author on the paper) said:
“By manipulating the amounts of key lipids within the cells we can tune protein production and so improve yields of protein biotherapeutic drugs. This could significantly improve the production of difficult-to-make proteins so that they can be made in sufficient quantities to be tested in the clinic. This will open up opportunities to develop new protein drugs for diseases where no good treatments are currently available. The research has been part of a long standing and successful collaboration with Lonza Biologics and is a standout example of how academia can work with industry to provide creative solutions for industrially relevant problems.”
The technology developed in this ground-breaking work has resulted in the filing of several new patents.
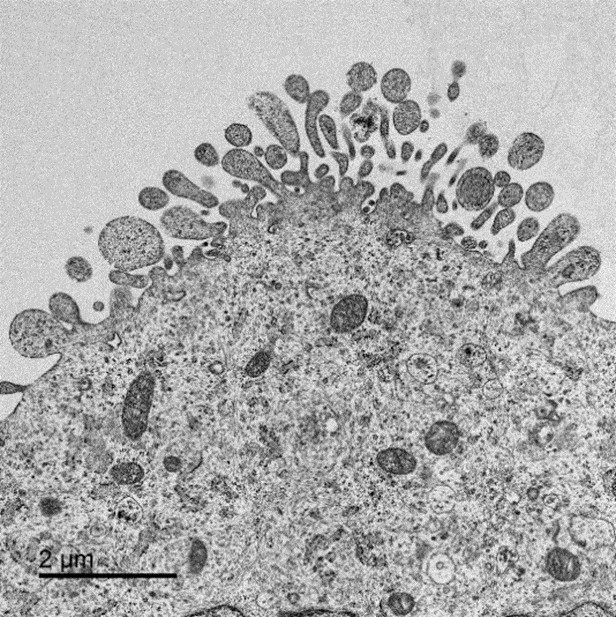
Image: Electron microscopy of a Chinese hamster ovary cell engineered to overexpress lipid modifying genes. The image shows an abundance of vesicle-like protrusions which are more prevalent in the engineered cell compared to a non-engineered control. The increase in vesicle budding is likely to result from an increase in membrane fluidity and may contribute to the increased capacity of these cells to make higher yields of protein biotherapeutic drugs.