Dr. Mark Howard, Reader in Biological NMR Spectroscopy, comments on a recently published article describing a new approach to studying proteins.
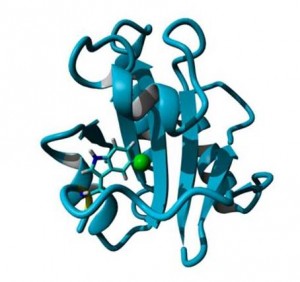 “Proteins are one of the most important biological molecules that exist and drive basic biology as well as being responsible for many cellular disease processes. My group designs new and interesting methods to study proteins in order to understand how they work. Our recent article describes how we have labeled a protein with a fluorine atom and watched how the properties of the fluorine atom changed as the protein binds a molecular ligand. Cell molecules communicate and function through these binding events between ligands and proteins and this is also the process by which pharmaceutical drug molecules interact with proteins to manage illnesses and diseases. Our method of using fluorine as a marker on the protein to follow this process provides a new approach to observing cell molecules and drug screening in action at the molecular level.”
“Proteins are one of the most important biological molecules that exist and drive basic biology as well as being responsible for many cellular disease processes. My group designs new and interesting methods to study proteins in order to understand how they work. Our recent article describes how we have labeled a protein with a fluorine atom and watched how the properties of the fluorine atom changed as the protein binds a molecular ligand. Cell molecules communicate and function through these binding events between ligands and proteins and this is also the process by which pharmaceutical drug molecules interact with proteins to manage illnesses and diseases. Our method of using fluorine as a marker on the protein to follow this process provides a new approach to observing cell molecules and drug screening in action at the molecular level.”
Dr. Howard is a key member of our teaching team, bringing his expertise to teaching the structure and function of proteins and the roles of magnetic resonance in biology and medicine.
Curtis-Marof, Doko, Rowe, Richards, Williamson and Howard: 19F NMR spectroscopy monitors ligand binding to recombinantly fluorine-labelled b’x from human protein disulphide isomerase (hPDI). Org. Biomol. Chem. (2014), 12, 3808.
